On Oct 4th, 2023, Shixian Lin’s Lab from Life Sciences Institute, Zhejiang University, published a review article in Accounts of Chemical Research entitled “New Strategies for Probing the Biological Functions of Protein Post-translational Modifications in Mammalian Cells with Genetic Code Expansion”.
Protein post-translational modification (PTM) is a major mechanism for functional diversification of the human genome and plays a crucial role in almost every aspect of cellular processes, and the dysregulation of the protein PTM network has been associated with a variety of human diseases. Using high-resolution mass spectrometry, protein PTMs can be efficiently discovered and profiled under various biological and physiological conditions. However, it is often challenging to address the biological function of PTMs with biochemical and mutagenesis-based approaches. Specifically, this field lacks methods that allow gain-of-function studies of protein PTMs to understand their functional consequences in living cells. In this context, the genetic code expansion (GCE) strategy has made tremendous progress in the direct installation of PTMs and their analogs in the form of non-canonical amino acids (ncAAs) for gain-of-function investigations. In addition to studying the biological functions of known protein PTMs, the discovery of new protein PTMs is even more challenging due to the lack of chemical information for designing specific enrichment methods. Genetically encoded ncAAs in the proteome can be used as specific baits to enrich and subsequently identify new PTMs by mass spectrometry.
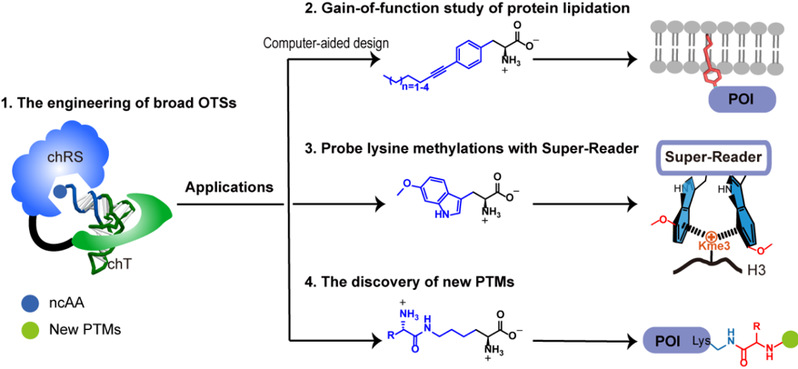
Figure 1. New strategies for probing the biological functions of protein PTMs in mammalian cells with GCE.
In this Account, the authors discuss their recent developments in the investigation of the biological functions of protein PTMs and the discovery of protein PTMs using new GCE strategies. First, the authors leveraged a chimeric design to construct several broadly orthogonal translation systems (OTSs). These broad OTSs can be engineered to efficiently incorporate different ncAAs in both E. coli and mammalian cells. With these broad OTSs: (1) They develop a computer-aided strategy for the design and genetic incorporation of length-tunable lipidation mimics. These lipidation mimics can fully recapitulate the biochemical properties of natural lipidation in membrane association for probing its biological functions on signaling proteins and in albumin binding for designing long-acting protein drugs; (2) They demonstrate that the binding affinity between histone methylations and their corresponding readers can be substantially increased with genetically encoded electron-rich Trp derivatives. These engineered affinity-enhanced readers can be applied to enrich, image and profile the interactome of chromatin methylations; (3) They report the identification and verification of a novel type of protein PTM, aminoacylated lysine ubiquitination, using genetically encoded PTM ncAAs as chemical probes. This approach provides a general strategy for the identification of unknown PTMs by increasing the abundance of PTM bait probes.
Dr. Wenlong Ding, Dr. Hongxia Zhao and Dr. Yulin Chen are the co-first authors, and Dr. Shixian Lin is the corresponding author in this study. This research was supported by the National Key R&D Program of China (Grant 2019YFA09006600), the National Natural Science Foundation of China (Grants 22222705, 92253302, 91953113, 22207095, and 22207096), and China Postdoctoral Science Foundation (grant 2019M652072 and 2022M712782).
Link: https://pubs.acs.org/doi/epdf/10.1021/acs.accounts.3c00460